Supplier Qualification Management (SQM) is a crucial practice for any company. However, the practice is still applied only partially and not always at the right time. What are the conditions for your company to make Supplier Qualification a genuine comprehensive and structured approach that enables to get the best value in a sustainable way?
Until recently, in many companies, supplier prequalification was frequently conducted at the time of the invitation to tender. The direct consequence in the specification phase of the sourcing process is a joyful mix – not to say a confusion – between (1) the functional/technical need related to the sourcing project and (2) the basic requirements the company is entitled to expect from its potential suppliers, even before the latter are invited to respond to a call for tenders where they must demonstrate their ability to cover the need.
Today things are changing. Any modern company qualifies its suppliers in a better organized way, following a real structured and clear process avoiding incidentally any confusion or mixing between the pre-qualification stage and the consultation stage. Supplier Qualification is thus the whole of the activities that the company conducts to constitute (pre-qualification) and maintain (re-qualification) a panel of suppliers which can then be consulted in the context of calls for tenders. Pre-qualification concerns potential suppliers of the company and Re-qualification concerns active suppliers of the company, ie those who are already under contract.
Incidentally, it should also be noted that having a solid supplier qualification process becomes a necessity for companies ISO9001 certified or undergoing certification.
Whatever their motivations, more and more companies have a real Supplier Qualification process, have a real Supplier Qualification Program to apply concretely this process, and the more advanced ones adopt a suited IT solution to structure their approach and make it more effective.
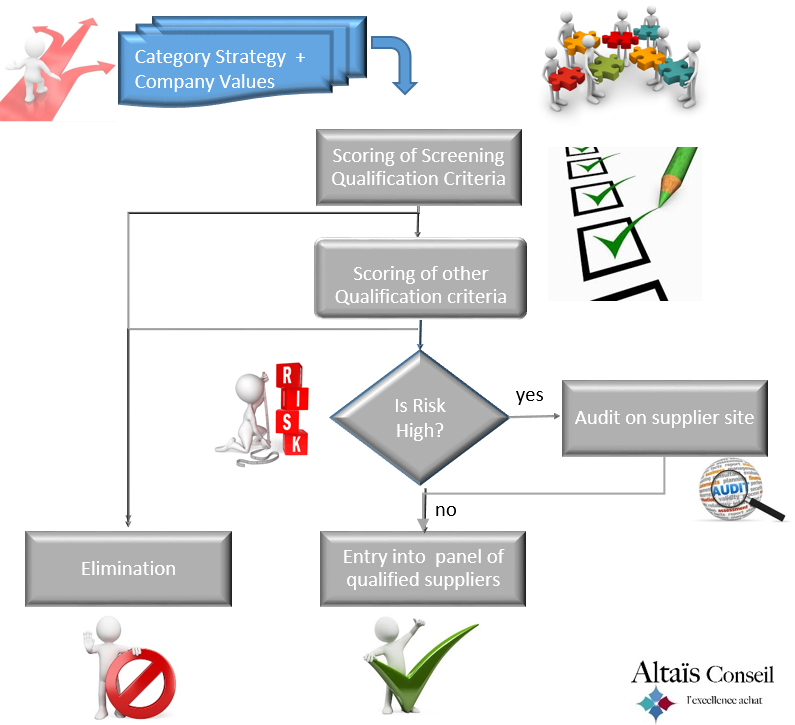
In summary, Qualifying a Supplier means following the below macro-steps:
- Assign to the supplier a risk class that depends from one hand on the type of product or service to be provided and from the other hand on the company’s risk analysis rules
- Evaluate supplier’s compliance with a set of qualification criteria defined at company level; this evaluation, which leads to a qualification score and to a qualification validity period, is carried out by analyzing the answers to a questionnaire sent to the supplier and – if required by the the level of risk – by completing this analysis through a detailed audit on supplier’s site.
- Identify the criteria for which the level of compliance (ie the qualification score) needs to be improved
- Define, for each identified criterion, the improvement action
- Monitor/control the implementation of the improvement plan
- Go to 1 periodically; the period depends on (1) the qualification score and on (2) the qualification risk class assigned to the supplier
That being said, what are the key factors for a sustainable success of your Supplier Qualification process?
- Always keep in mind that you will have the suppliers you deserve.
- Explain to your board that by mastering supplier qualification, you act upstream to help create value for your company.
- Explain to your board that controlling supplier qualification will contribute to Control Supplier Risk for your company.
- Resolutely base your qualification process on Risk; this is always crucial and even more crucial for international procurement especially when the suppliers to be pre-qualified are located in Low Cost Countries.
- Always bear in mind that this is a continuous improvement process to be carried out in full, i.e following the above mentioned macro-steps.
- Use an efficient but simple qualification framework; do not reinvent the wheel, remember that even if there is no standard for supplier qualification criteria, the most common repositories cover 7 sections (Health Safety Environment, Finance, Ethics, Legal, Human Resources Management , Quality Management and Continuous Improvement, Supplier Services & References); Weigh the sections & criteria according to the context of your company.
- Make sure that this repository is defined at company level, therefore common to all suppliers; you will gain some more credibility vis-à-vis your suppliers and your internal customers.
- Do not hesitate to define screening criteria and ensure that they are related to the fundamental values and basic requirements of the company (eg ethics or health & safety); the idea being that if the potential supplier does not meet one of the screening criteria then the qualification process is stopped, the other qualification criteria are not looked at and the supplier is eliminated; you will optimize the effectiveness of your approach and ensure your credibility.
- Opt for a continuous improvement rating system; a rating scale ranging from 1 (basic conformity to qualification requirements) to 5 (excellent conformity) is particularly well adapted; using such a rating system enables you to assign a qualification score to each supplier, this score will be re-evaluated when the supplier will need to be re-qualified, for example after one year or two years of contract according to the classification risk class that will be assigned.
- Involve your buyers team; their engagement is essential, each buyer will feel all the more responsible for the complete management of the qualification of all its suppliers.
- Rely on your quality manager; if there is one in your company, the quality manager will be a great ally to support your approach especially if your company is certified or pending certification (ISO9001 for example)
- Focus your efforts on your key suppliers; you cannot do everything, « do not bite off than you can chew »
- Establish a multidisciplinary Qualification team rather than entrusting the approach to only one person; that way, you will reduce the risk of subjectivity, and avoid, in particular, that a given criterion in a given qualification area (eg HSE area) is evaluated by a non competent person; this way of proceeding will also give all the actors of the company the strong feeling of having been involved in the constitution of the panel of qualified suppliers, all the more so since the strategy mentions that it was necessary to « source » internationally.
- Perform a qualification audit only if it the level of risk is significant; such a detailed qualification audit on supplier’s site consists of verifying the supplier information collected during the questionnaire phase; this certainly reduces the above mentioned subjectivity, however it will have real added value only if the associated level of risk requires it; keep your energy and resources for suppliers at significant risk.
- Spread your yearly re-qualification campaigns over the year; i.e do not concentrate them on a single period, thus you will spare the agendas of your internal customers so that the process is not seen as a constraint
- Adopt a suitable IT solution; this is undoubtedly the best way: to structure your approach, to effectively share data with your internal customers and with your suppliers, to ensure data integrity and also, as appropriate, to instantly prove the soundness of the SQM process to any quality/ISO9001 auditor (Chapter 7.4 / Section 7.4.1.)
And of course, Have Fun!
To receive new Excellence-Achat Blog posts directly to your inbox, subscribe using the contact form in the topbar of this page (we never share your e-mail), or send a note to contact@altais-conseil.com